The Curious Case of the Shape-Shifting Liquid: A Happy Accident in the Lab
Imagine shaking a bottle of salad dressing and watching it always settle back into the shape of a Grecian urn. Not just once, but every time. That’s not a scene from a fantasy hollywood film. It’s what actually happened in a university lab, and it’s left scientists scratching their heads and rethinking some fundamental ideas about physics.
This isn’t just a scientific curiosity. It’s a discovery that may someday impact everything from smart materials to biomedical devices. But what makes this so remarkable isn’t just the result but how it was found, almost by mistake.
How a Student’s Curiosity Challenged the Textbook
Anthony Raykh, a graduate student in polymer science at the University of Massachusetts Amherst, wasn’t trying to rewrite laws of physics. He was simply experimenting with a mix of oil, water, and nickel particles. This specific combo usually behaves predictably. But what he saw defied team’s expectations.
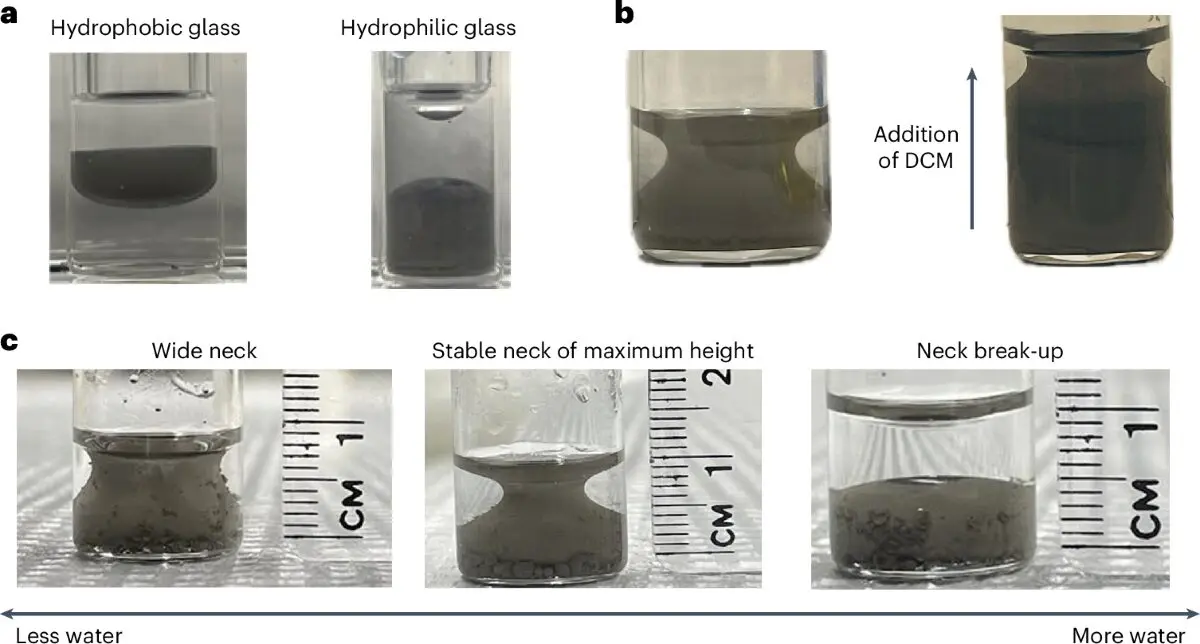
After shaking the mixture to create an emulsion (a temporary blend of liquids that typically don’t mix), Raykh noticed something weird. Instead of separating into two layers like oil and vinegar do, the mixture took on a stable, symmetrical shape. And not just any shape it looked like a miniature Grecian urn, elegant and constant. Even after repeated shaking, the mixture always found its way back to that same urn-like form. It seemed almost alive, like it remembered its shape. Mind Boggling phenomenon. Right?
Why That’s So Weird
To understand the shock among scientists, you need to understand a bit about thermodynamics, the science of heat, energy, and how systems naturally evolve. Normally, when liquids that don’t mix (like oil and water) are left alone, they settle into shapes that minimize surface area. This is nature’s way of conserving energy.
The round droplets you see in vinaigrette or milk are nature’s go-to shape for this: spheres have the smallest possible surface area for a given volume. But the Grecian urn? It has a much higher surface area, which seems to go against this thermodynamic rulebook.Woah!
Dr. Thomas Russell, a seasoned professor working with Raykh, called it “really odd.” And in science, odd is often a good thing.
The Invisible Forces Behind the Magic
So what’s causing this defiant behavior? The answer lies in the magnetic personality of the nickel particles. These tiny metallic bits aren’t just floating around aimlessly. Under the right conditions, they form magnetic dipoles (north-south pole pairings) which interact and create organized chains in the mixture.
These magnetic forces are strong enough to dominate how the oil and water separate, sculpting the entire emulsion into that Grecian urn. It’s a tug-of-war between the laws of thermodynamics and the pull of magnetism. In this strange case, magnetism wins.
Rather than truly breaking the laws of physics, the researchers realized they were observing a rare loophole. The thermodynamic rules still hold when applied to the whole system. However, the local interactions i.e the tiny magnetic dances between particles produce this weird outcome.
More Than a Lab Trick: Why It Matters
It’s tempting to file this under “cool but useless,” but this discovery could have serious implications. Materials that “remember” their shape are highly valuable in fields like soft robotics, medicine, and self-healing materials.
Imagine a surgical device that can twist into shape when needed or a material that naturally reforms if it’s damaged. All because of these micro-level particle interactions. It also highlights that even well-studied systems like oil and water can still reveal surprises in emergent behavior, where simple rules produce complex outcomes.
Science by Serendipity
Raykh didn’t set out to challenge the fundamentals of energy and motion. His discovery came through observation, curiosity, and most importantly not ignoring something strange just because it didn’t fit expectations. This story is a reminder that science isn’t just about following formulas, it’s about noticing when something weird happens and asking why. Sometimes, the path to groundbreaking insights begins with a simple shake of a bottle.
A World Waiting to Be Stirred
Who would’ve thought that a mixture of oil, water, and metal dust could hint at a new understanding of matter? As this young scientist’s finding shows, the universe is still full of surprises. Sometimes, you just have to stir things up to find them.
Source: Nature