The reason behind paper’s easy tearing when wet

The reason why paper tears more easily when it’s wet is due to its chemical structure, specifically the hydrogen bonds present in the paper. If you’ve ever experienced the frustration of spilling a drink on your desk or placing a dinner napkin on a damp surface, you know how easily paper becomes flimsy when wet. Even a small droplet of water can weaken a pristine sheet of paper. But what causes paper to tear more easily when wet? The answer lies in the chemical structure of paper.
Cellulose Fibers: The Building Blocks of Paper
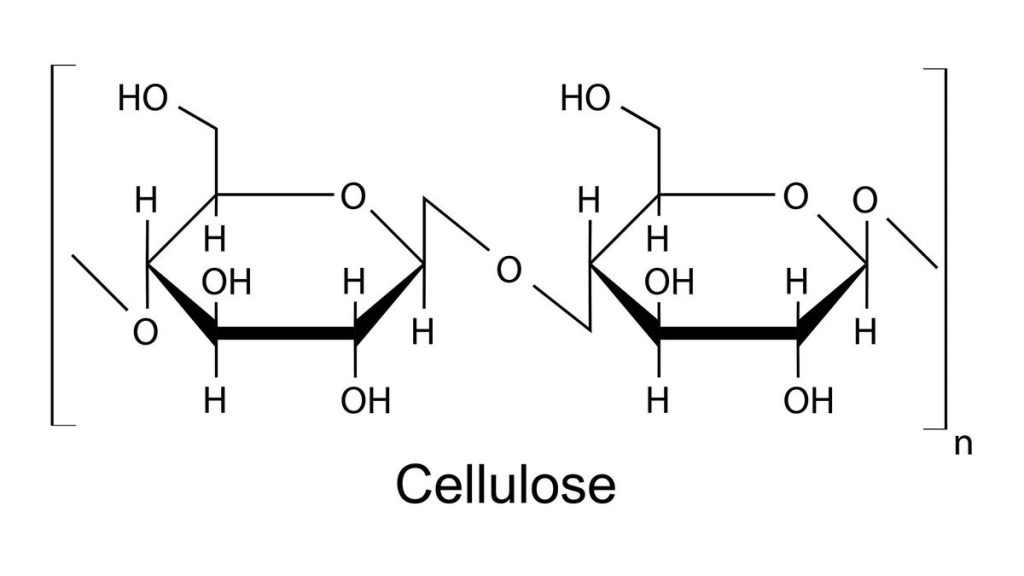
Paper is primarily made up of cellulose fibers, which are natural polymer molecules derived from wood. These fibers are interlocked with each other through hook-like irregularities on each strand of cellulose. Additionally, they are bonded to each other by hydrogen bonds. Hydrogen bonds are crucial in chemistry, as they are responsible for many important interactions. These bonds occur between molecules with one end slightly positive and the other slightly negative. The positive end of one molecule is attracted to the negative end of another nearby molecule, resulting in their connection.
Glue that Holds Paper Together
Molecules that contain oxygen bonded to hydrogen, such as water (H2O), are particularly susceptible to hydrogen bonding. The cellulose polymer, which makes up the structure of paper, is covered in oxygen-hydrogen handles along its entire length. When a piece of dry paper is torn, overcoming the intermolecular forces, friction, and fiber entanglements is necessary. However, when paper becomes wet, the fiber matrix swells and the fibers start to detach, causing a loss of strength and making it easier to tear.
Weakening Under Wet Conditions
On a chemical level, the presence of water disrupts the hydrogen bonds that hold the cellulose fibers together. Water, containing its own oxygen-hydrogen bond, begins to form hydrogen bonds with the cellulose, preventing the other fibers from binding. With fewer interactions between the cellulose polymers, it becomes easier to separate the fibers, hence requiring less force to tear the paper.
Variability in Paper Types
It is important to note that not all paper is the same. Various paper products, such as toilet paper, paper towels, newspapers, printer paper, and cardboard, may have different properties despite sharing almost identical cellulose fibers. This variation is because of the additional additives included during the papermaking process. Paper manufacturers utilize chemical techniques to enhance the properties of paper products, with strength being a significant focus.
Dry strength additives, like potato starch, are used to strengthen the fiber matrix in paper products such as packaging boxes. A layer of this natural compound is applied to the paper’s surface as a gel and forms a toughened barrier around the interwoven cellulose fibers when dried. This starch surface acts as scaffolding, greatly boosting the paper’s strength. However, even with reinforced cardboard, exposure to moisture can still have damaging effects. Starch dissolves in water, causing the added strength to rapidly deteriorate when the paper gets wet.