Metal, Mind, and Molecules: The Story Behind the 2025 Nobel Prize in Chemistry
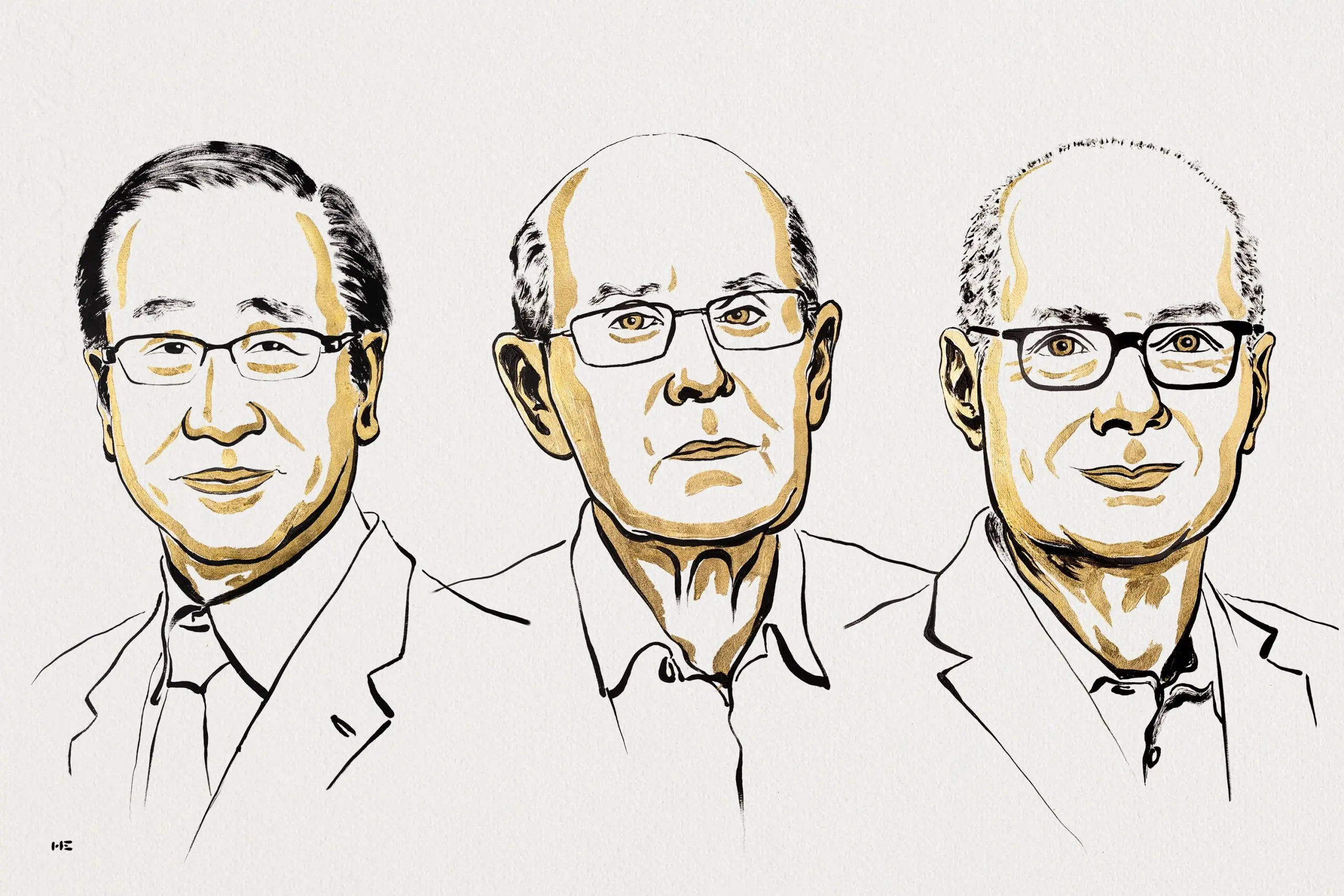
On 8 October 2025 the Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry to Susumu Kitagawa (Kyoto University), Richard Robson (University of Melbourne) and Omar M. Yaghi (University of California, Berkeley). The trio was honoured “for the development of metal–organic frameworks” (MOFs) — a family of crystalline, porous materials that chemists today use as tailor-made, molecular-scale sponges and reaction vessels. The Academy described the winners’ work as the creation of “new rooms for chemistry” — engineered internal spaces where gases, liquids and guest molecules can be stored, separated, transformed or harvested.
This award recognises a decades-long revolution in materials chemistry. MOFs are now used across energy and environmental problems: from capturing CO₂ and storing hydrogen to purifying water, harvesting moisture from desert air and enabling new catalytic processes. And they point to entirely new ways of designing functional materials by construction, not merely by discovery.
What exactly is a metal–organic framework?
At its heart a MOF is a crystal built from two complementary parts:
- Nodes (metal ions or metal clusters). Think of these as the corners in a lattice. Single metal ions (e.g., copper, zinc) or small clusters of metal atoms act as connecting hubs.
- Linkers (organic molecules). Long, rigid organic molecules (typically aromatic carboxylates, azoles or related ligands) act as struts that connect the metal nodes in a regular pattern.
When nodes and linkers are chosen and arranged correctly they self-assemble into an extended network with an open, highly regular geometry. Crucially, this geometry produces large internal cavities and channels — the “rooms” in which other molecules can reside or move. Because the building blocks are modular, chemists can vary metals, linkers and connectivity to tune pore size, chemical environment, stability and function.
Two technical points that explain why MOFs are scientifically exciting:
- Extreme internal surface area. Some MOFs have surface areas measured in thousands of square metres per gram — meaning tiny amounts of material present an enormous internal surface, ideal for adsorption and storage.
- Reticular (design-by-construction) chemistry. Rather than relying on chance for useful structures, reticular chemistry (pioneered by Yaghi) treats molecular building blocks like Lego pieces: predictable bonding geometries let chemists design targeted topologies and functions. This shifts materials chemistry from discovery to rational design.
How MOFs are made
Typical MOF synthesis is deceptively simple in concept: dissolve a metal salt and an organic linker in a solvent (sometimes under heat or pressure) and allow them to assemble into crystalline frameworks. But the art is in control: solvent, concentration, temperature, pH and the nature of the metal cluster determine which topology and pore architecture emerge. Over the years researchers have developed methods to produce highly stable MOFs (for example, zirconium-based frameworks that resist water and heat) and strategies to introduce catalytic sites, functional groups or hierarchical porosity. Those synthetic advances have been essential to moving MOFs from lab curiosities to real applications.
Why the discovery matters
MOFs are now more than a materials novelty; they are solving concrete problems:
- Gas capture and climate mitigation. MOFs can selectively adsorb CO₂ from gas streams and, in some pilot projects, help lower emissions from industrial sources. Their tunable chemistry allows high selectivity even in mixed-gas environments.
- Water harvesting from air. Certain MOFs can capture water vapor at low humidity and release it upon mild heating — a method that has been demonstrated to produce potable water from arid air, with promising prototypes aimed at communities with scarce fresh water.
- Energy storage and separations. MOFs are being tested to store hydrogen and other energy-carrying gases and to separate complex mixtures (for example, removing pollutants or “forever chemicals” from water). Their high surface areas, combined with tailored pore chemistry, make them uniquely useful for separations that are otherwise energy-intensive.
- Catalysis and sensors. By installing active sites within their pores, MOFs can catalyse reactions selectively or act as highly sensitive sensors because binding events inside pores produce measurable signals.
These applications are already moving beyond bench demonstrations: industrial partnerships, pilot plants and startup activity have accelerated in the past decade — precisely the kind of societal reach that the Nobel committee often recognises.
Who did what and why they share the prize
MOFs emerged through contributions from many groups over time; the 2025 Nobel honours three pioneers whose work shaped the field in complementary ways.
- Richard Robson (b. 1937) — Robson is widely credited with foundational work in coordination polymers and early MOF-like networks (dating from the 1980s and 1990s). His crystal-engineering insights clarified how transition-metal centres and organic ligands could be used to build extended, infinite polymeric frameworks. Robson’s early conceptual and synthetic groundwork helped show that predictable extended architectures were possible.
- Susumu Kitagawa (b. 1951) — Kitagawa built MOF chemistry into a broad, experimentally driven discipline. He and collaborators demonstrated porous coordination polymers with guest-responsive behaviour and helped establish that such frameworks could be dynamic — changing their pore environments when guests enter or in response to stimuli — which opened routes to selective separations, sensing and responsive materials. Kitagawa’s experiments made porous frameworks an experimental mainstay.
- Omar M. Yaghi (b. 1965) — Yaghi coined and developed reticular chemistry, the idea of stitching molecular building blocks into predictable extended structures (both MOFs and covalent organic frameworks, COFs). He pushed MOFs toward practical stability and function: synthesising robust frameworks, demonstrating extraordinarily high surface areas, and championing applications like water harvesting and gas storage. Yaghi’s laboratory also expanded the chemical toolbox for MOF design and made reticular methods widely accessible to other researchers.
The Nobel committee’s citation — “for the development of metal–organic frameworks” — recognises that the field is the product of complementary theoretical vision, synthetic mastery and experimental application. Each laureate’s contributions helped turn an idea into a global research field with tangible societal potential.
Three portraits: life and work
Omar M. Yaghi — reticular chemistry and robust MOFs
Born in Amman (1965), Omar Yaghi trained in the US and over the last three decades established reticular chemistry as a distinct approach to materials design. He held positions at several institutions and is a long-time faculty member at UC Berkeley. Yaghi’s group produced highly stable frameworks (including zirconium-based MOFs) and pushed MOFs into real-world applications such as water harvesting and CO₂ capture. He has also been a vocal builder of the field — mentoring many students and pushing commercial translation. Berkeley News

Susumu Kitagawa — experiments that revealed porous crystals are functional
A Kyoto native, Kitagawa has been a central figure in Japan’s materials and coordination-chemistry community. He demonstrated that porous coordination polymers can be dynamic and guest-responsive, and his laboratory developed experimental methods to characterise how molecules move in and out of frameworks — knowledge that underpins separations and sensing applications today. Kitagawa’s work emphasised the experimental richness of porous frameworks.
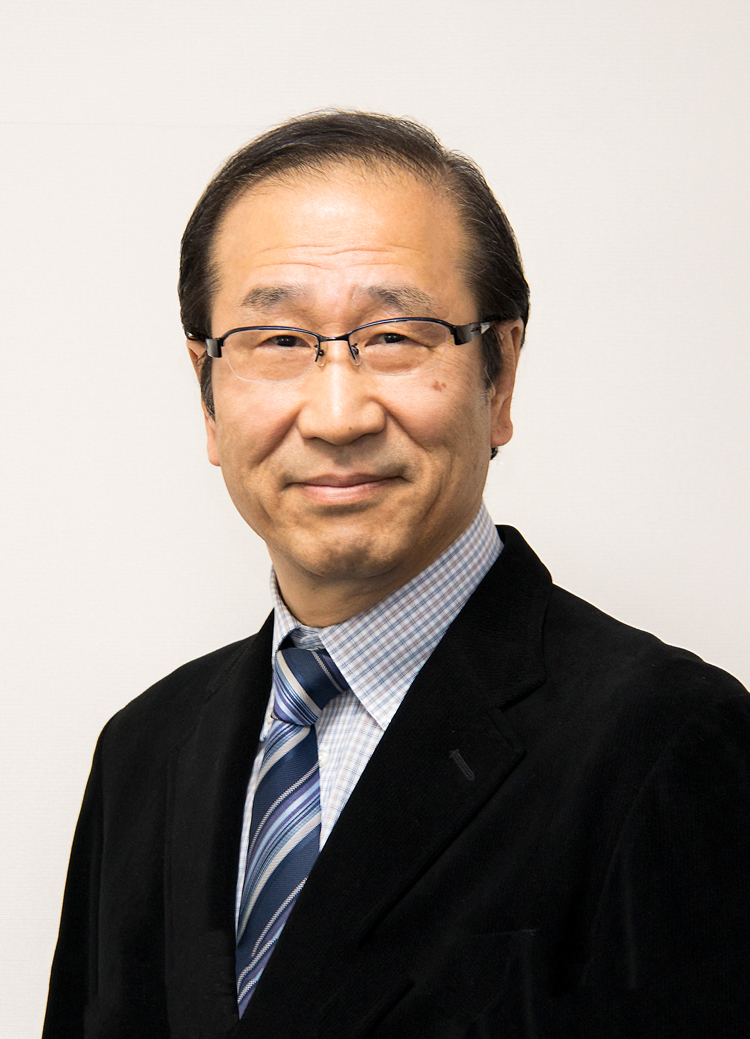
Richard Robson — early crystal engineering and coordination networks
Robson’s career spans decades at the University of Melbourne. He made pioneering contributions to coordination polymers and crystal engineering that prefigured the MOF concept. His early structural ideas and synthetic strategies laid a conceptual foundation, showing how simple coordination chemistry could be extended into infinite networks with predictable patterns. Robson has been a steady influence in the field since the late 20th century.

(For concise biographical facts, the Nobel Foundation’s laureate pages are the authoritative source and provide interviews, photo galleries and publication lists for each laureate.)
Challenges, criticisms and next steps
MOFs are not a panacea. Practical deployment raises real-world engineering questions:
- Stability in real environments. Many MOFs are moisture-sensitive; creating frameworks that remain intact and functional in humid, hot or chemically harsh conditions remains a key engineering challenge. Advances (for example, robust zirconium MOFs) have improved stability, but scale-up and longevity testing are ongoing.
- Economics and scale. Producing MOFs at industrial scale, and doing so in an energy- and cost-efficient way, is still an area of active development. For global climate impact, materials must be affordable, durable and recyclable.
- Environmental lifecycle. The environmental footprint of MOF manufacture and disposal is under scrutiny: researchers are studying greener synthesis routes and recycling strategies.
Despite these hurdles, the field’s rapid progress — from elegant lab crystals to pilot projects and commercial interest — shows MOFs are moving fast along the innovation curve. The Nobel prize highlights both the fundamental science and its societal promise.
Why this Nobel matters to science and society
The 2025 prize is notable because it rewards a materials concept that is both fundamental and practical. MOFs embody a shift toward rational materials design — building function into structure — and they address problems that matter today: clean water, cleaner industrial processes and emissions mitigation. By celebrating the architects of this molecular architecture, the Nobel committee underscored how careful, curiosity-driven chemistry can produce platforms that engineers and society can use to tackle grand challenges.
Further reading (authoritative, recent)
- Nobel Prize in Chemistry 2025 — press release and laureate pages.
- Nature and Science coverage of the 2025 award and MOF developments.
- Reviews and feature-stories about MOF applications (Scientific American) that summarise water-harvesting, CO₂ capture and industrial prospects.






